carbon valence electrons
119 rows Valence electrons. Their electronic configuration goes about.
 |
| Atoms Are The Fundamental Unit Of Matter And Composed Carbon Bohr Model With Valence Electrons Hd Png Download Transparent Png Image Pngitem |
The 2 nd energy level contains 2s and 2p.
. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. Carbon is an element with. To find the total valence electron in CO2 look at the periodic group of carbon and oxygen atoms. In chemistry and physics a valence electron is an electron in the outer shell associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not.
The significance of carbon having four valence electrons is so that its outermost shell can form up to four covalent bonds. CARBON AS AN ELEMENT. Carbons electronic configuration C 6 is 1 s 2 2 s 2 2 p 2. Answer 1 of 4.
By looking at the periodic table we come to know carbon belongs to 14 groups and oxygen. Answer 1 of 2. An elements valency is a measure of its ability to combine with other atoms to form chemical compounds or molecules. Valence electrons in simple words are the electrons revolving continuously in the outermost shell or orbit of an atom.
There are 4 valence electrons 2s 2 2p 2 in the outermost shell of the carbon atom. The outermost shell or the. 13 rows Carbon is a classified nonmetallic element and its symbol is C. Strictly speaking carbon has 3 valence electrons one unpaired in each p orbital.
When it bonds only with hydrogen it forms compounds called. For neutral atoms the number of valence electrons is equal to the atoms main group number. To find out the valence electrons of Carbon you have to see the position of carbon in the periodic table. To determine the number of valence electrons for CO2 the Carbon dioxide molecule well use the Periodic Table.
Carbon is the 6th element of the. Oxygen has the atomic number 8 and carbon has the atomic number 6. In the above electron configuration the highest energy level 2 is marked with green color. More specifically you have to see the group wise position of Carbon.
Organizing the Periodic Table by Group ski. The chemical symbol for Carbon is C. 1s2 2s2 2p2 So the number. For Lewis structure of CO2 you will now have two Oxygen atoms forming double bonds with a Carbon atom.
In carbene the carbon atom is bonded to the two atoms. The first easiest methods for finding the number of valence electrons for carbon atom or any different atoms first look at the periodic table if you properly look at the. The valence electron of the carbon can be determined using a Periodic Table. Finally count electrons of that energy level.
As all the valence electrons of all the atoms are used there are no. The main group number for an element can be found from its column on the periodic. Carbon has four valence electrons so it can achieve a full outer energy level by forming four covalent bonds. The carbon has the 4 electrons in its valence shelltext n 2.
These are covalent bonds formed by sharing the electron. 1s2 2s2 2p4 Carbon. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured thus its atomic mass is exactly 12 daltons by definition. However since the energy difference between its s orbital and the p orbital is small carbon.
Carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.
 |
| How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Covalent Bonding Nomenclature Chemistry |
 |
| How Many Valence Electrons Does Carbon Have |
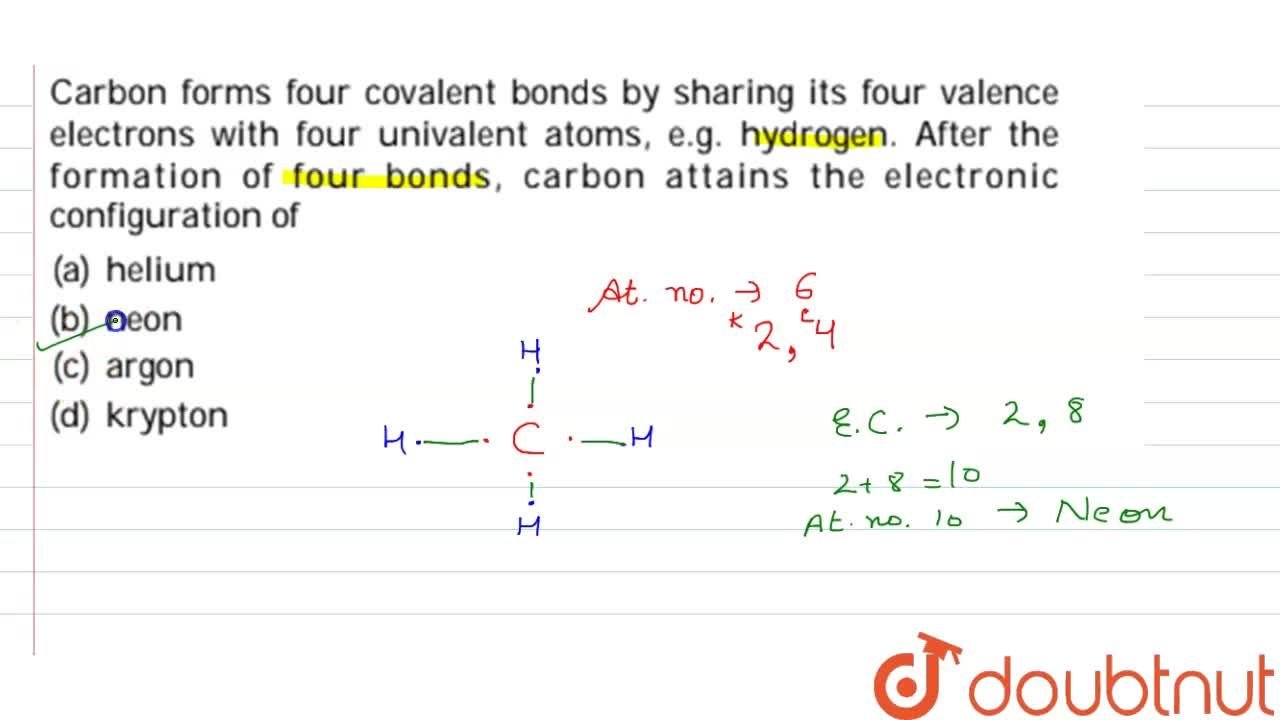 |
| Carbon Forms Four Covalent Bonds By Sharing Its Four Valence Electrons With Four Univalent Atoms E G Hydrogen After The Formation Of Four Bonds Carbon Attains The Electronic Configuration Of |
 |
| 4 The Behaviour Of The Valence Electrons In Carbon As A Carbon Atom Is Download Scientific Diagram |

Posting Komentar untuk "carbon valence electrons"